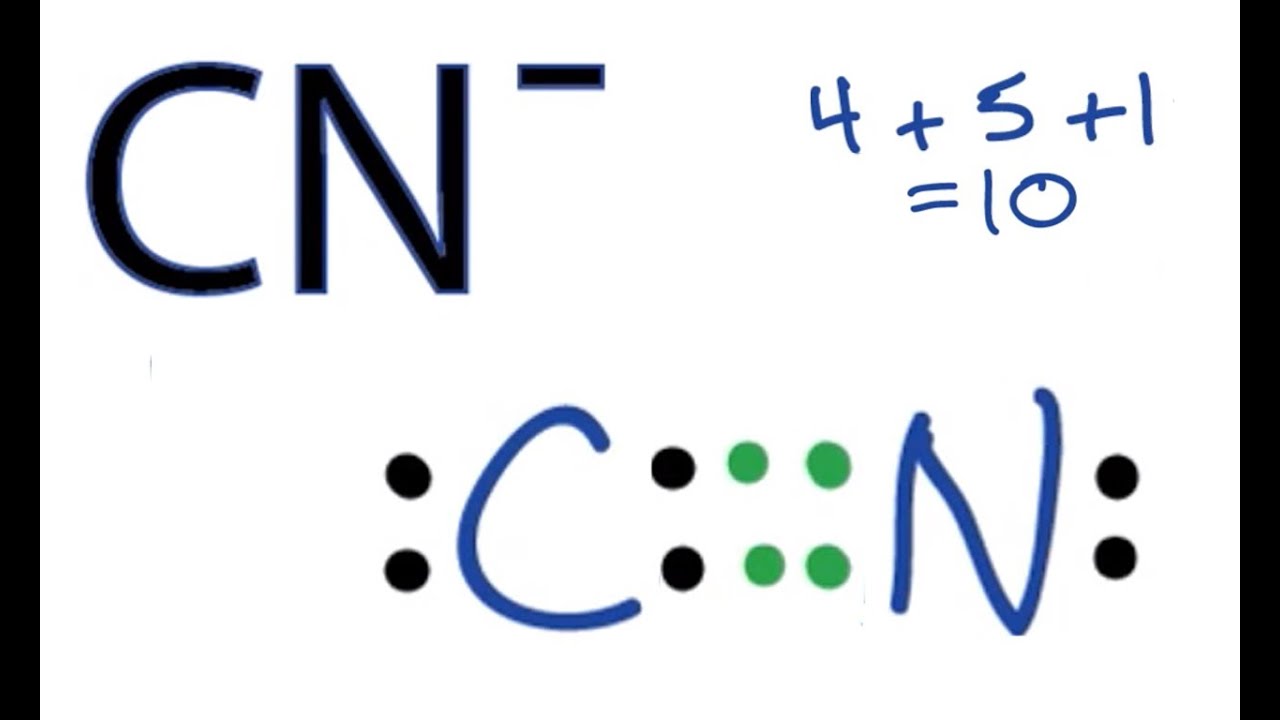Have you ever looked at a chemical formula like CN and wondered what it actually looks like on a molecular level? It's pretty cool, you know, how chemists can represent atoms and their connections using just dots and lines. That's where Lewis structures come in, and they're really helpful for seeing how electrons are arranged in a molecule, which, as my text points out, gives us a better grasp of what's going on with all the bond pairs and lone pairs that are present.
Figuring out the Lewis structure for something like the cyanide ion (CN-) or even the neutral CN molecule can seem a bit tricky at first, but it’s actually a very logical process. We're going to break it down, step by step, so you can see exactly how these atoms connect and share their electron bits. It's kind of like solving a puzzle, honestly, where each piece, each electron, has its own special spot.
We'll walk through not just the neutral CN, but also its charged relatives, CN+ and CN-. This will give you a pretty good idea of how adding or taking away an electron changes the whole picture. So, let's get ready to draw some dots and dashes and really get a handle on the cn lewis structure, because it’s a fundamental concept in chemistry.
- People Got To Be Free Lyrics
- Simsbury Vet
- Kate Winslet Titanic Paint
- Quotes From Zendaya
- Project Pat Wife
Table of Contents
- What Are Lewis Structures Anyway?
- Counting Valence Electrons: The Essential First Step
- Drawing the Neutral CN Lewis Structure
- Drawing the CN- (Cyanide Ion) Lewis Structure
- Drawing the CN+ (Cyanonium Ion) Lewis Structure
- Why These Structures Are So Important
- Frequently Asked Questions About CN Lewis Structures
What Are Lewis Structures Anyway?
Lewis structures, or electron dot representations, are like simplified maps of molecules. They show us how atoms are connected and where all the valence electrons are located, whether they are involved in bonds or just hanging out as lone pairs. My text mentions that Lewis gave us this electron dot representation of a molecule, which is very helpful for better understanding a molecule and knowing about all the bond pairs and lone pairs present with each atom. It's a way to visualize the electron sharing that happens when atoms come together to form compounds, which is pretty neat.
The main goal when drawing these structures is to make sure each atom, if possible, achieves a stable electron configuration, usually eight valence electrons around it, often called an octet. Hydrogen, for example, is happy with just two electrons. So, when you’re drawing, you’re essentially distributing those available valence electrons in a way that makes everyone, every atom that is, feel complete.
Counting Valence Electrons: The Essential First Step
Before you draw anything, the very first thing you need to do is count up all the valence electrons for the molecule or ion you’re working with. These are the electrons in the outermost shell of an atom, and they’re the ones that get involved in chemical bonding. It’s a bit like taking an inventory of all your building blocks before you start construction, you know. This total count will tell you how many dots you have to work with, essentially.
- Priyanka Quantico
- Alex Van Halen 2024
- Nick Nolte Sexiest Man Alive
- Gypsy Rose Facebook Posts
- Jordan Wiseley Movies And Tv Shows
Carbon and Nitrogen: Their Electron Contributions
Let's consider carbon and nitrogen, the two atoms in our cn lewis structure examples. Carbon, being in Group 14 of the periodic table, brings four valence electrons to the table. Nitrogen, in Group 15, contributes five valence electrons. So, for a neutral CN molecule, you just add those up: 4 + 5 = 9 valence electrons. This is your starting number, and it’s actually a bit unusual to have an odd number of electrons, which we’ll see can lead to some interesting structures.
When you have an ion, you adjust this count. For a negative charge, you add electrons. For a positive charge, you subtract them. For instance, my text asks, "How many valence electrons does the cyanide ion (CN) have?" Well, the cyanide ion is CN-, meaning it has an extra electron. So, for CN-, it's 4 (from C) + 5 (from N) + 1 (for the negative charge) = 10 valence electrons. This is a very common ion, and understanding its electron count is pretty important.
Conversely, for CN+, the cyanonium ion, you'd subtract an electron. That would be 4 (from C) + 5 (from N) - 1 (for the positive charge) = 8 valence electrons. Each of these different electron counts leads to a distinct Lewis structure, as we will see, which is quite interesting.
Drawing the Neutral CN Lewis Structure
Drawing the neutral CN molecule is a good place to start, as it helps us build up to the more common ions. Remember, we have 9 valence electrons to distribute. This molecule is a bit of an exception to the octet rule because of that odd number, which is something you'll sometimes encounter in chemistry, actually.
Step-by-Step for CN
Count Total Valence Electrons: As we just figured out, Carbon (4) + Nitrogen (5) = 9 valence electrons. This is our total pool of electrons, so to speak.
Place Atoms and Draw a Single Bond: Since there are only two atoms, Carbon and Nitrogen, just put them next to each other and connect them with a single dash, which represents one shared pair of electrons (2 electrons). So, C-N. This uses up 2 of our 9 electrons, leaving us with 7 electrons still to place.
Distribute Remaining Electrons as Lone Pairs: Now, you place the remaining electrons around the atoms to satisfy their octets, starting with the more electronegative atom, which is nitrogen. If you put 6 electrons around nitrogen (3 lone pairs), it now has 2 (from the bond) + 6 = 8 electrons, completing its octet. You have 7 - 6 = 1 electron left. That single electron goes on the carbon atom. So, you'd have C with one dot, single bond to N with three lone pairs. This is a very common way to approach these structures.
Form Multiple Bonds if Needed: In this case, nitrogen has an octet, but carbon only has 2 (from the bond) + 1 (lone electron) = 3 electrons. It needs more. To try and give carbon more electrons, you could move a lone pair from nitrogen to form a double bond. If you do that, nitrogen would still have two lone pairs and now be part of a double bond. Carbon would have 1 (lone electron) + 4 (from double bond) = 5 electrons. This still isn't an octet for carbon, and it also leaves us with an odd electron. This structure, C with one lone electron and a double bond to N with two lone pairs, is one possible representation, but it's important to remember the odd electron. It's not always about perfect octets, especially with odd electron species.
Checking Octets and Formal Charges for CN
For neutral CN, because of the odd number of electrons, neither atom can truly achieve a full octet. This is an example of a radical. The most stable form usually has the odd electron on the less electronegative atom, which is carbon. So, you might see C with one lone electron, triple bonded to N, which has one lone pair. Carbon would then have 1 + 6 = 7 electrons, and nitrogen would have 2 + 6 = 8 electrons. This is one common way to represent it, and it's a bit of an exception, as a matter of fact, to the usual octet rule.
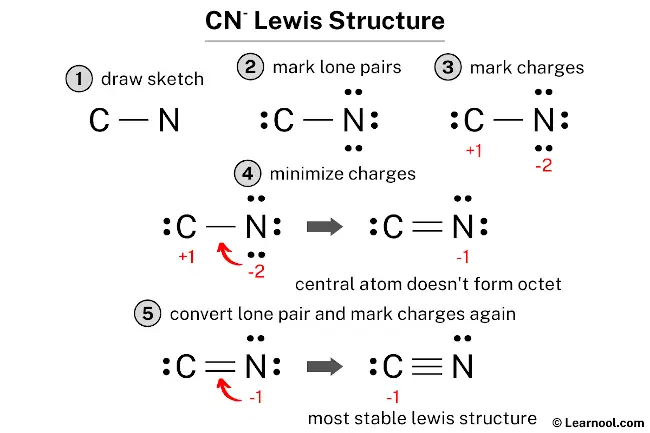
Drawing the CN- (Cyanide Ion) Lewis Structure
The cyanide ion, CN-, is probably the most frequently encountered of the three. My text states, "The lewis dot structure of cyanide ion starts with a c atom connected to a n atom with three dashes." It also mentions, "On the opposite sides on each atom are two dots for the unshared valence." This gives us a really strong hint about the final structure, which is helpful.
Valence Electrons for CN-
Let's quickly re-confirm the valence electron count. Carbon contributes 4, Nitrogen contributes 5, and the negative charge means we add 1 extra electron. So, 4 + 5 + 1 = 10 valence electrons. This even number makes achieving octets much more straightforward, so that's good news.
Step-by-Step for CN-
Count Total Valence Electrons: As determined, 10 valence electrons.
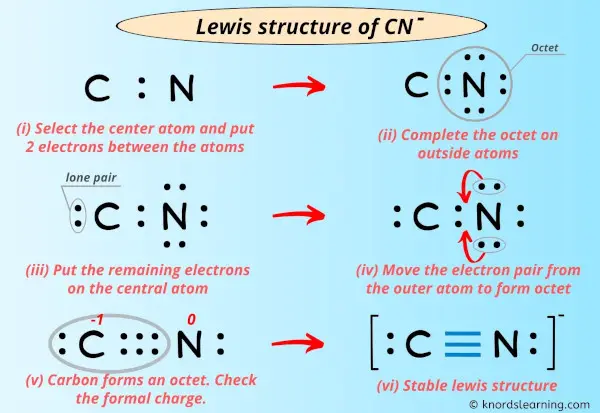
Place Atoms and Draw a Single Bond: Put C and N side by side and connect them with a single bond (C-N). This uses 2 electrons, leaving 8 electrons to place.
Distribute Remaining Electrons as Lone Pairs: Start by giving lone pairs to the more electronegative atom, nitrogen, until its octet is full. Nitrogen already has 2 electrons from the single bond. So, add 6 more electrons as 3 lone pairs around nitrogen. This makes nitrogen have 8 electrons (2 from bond + 6 lone pair electrons). We have used 2 (bond) + 6 (lone pairs) = 8 electrons so far. We started with 10, so 10 - 8 = 2 electrons remaining. These last 2 electrons go on the carbon atom as one lone pair. At this point, carbon has 2 (from bond) + 2 (lone pair) = 4 electrons. Nitrogen has 8 electrons. Carbon needs more electrons to reach an octet, which is pretty clear.
Form Multiple Bonds: To give carbon an octet, we need to move lone pairs from nitrogen to form multiple bonds. If we move one lone pair from nitrogen to form a double bond, nitrogen would then have 4 electrons in bonds and 4 in lone pairs (2 lone pairs). Carbon would have 4 electrons in bonds and 2 in lone pairs. Carbon still needs more. If we move *another* lone pair from nitrogen, forming a triple bond, nitrogen will have 6 electrons in bonds and 2 in a lone pair (1 lone pair). Carbon will have 6 electrons in bonds and 2 in a lone pair. Now, both carbon and nitrogen have 8 electrons! This is the stable arrangement, and it's a very common structure, actually.
Final Touches: Lone Pairs and Formal Charges for CN-
The final structure for CN- looks like this: C with one lone pair, triple bonded to N with one lone pair. My text said, "The lewis dot structure of cyanide ion starts with a c atom connected to a n atom with three dashes," and "On the opposite sides on each atom are two dots for the unshared valence." This perfectly matches our triple bond and one lone pair on each atom. Now, let's check the formal charges, which is a way to see how the electrons are distributed among the atoms in the structure. It’s pretty important for understanding stability.
Formal Charge on Carbon: Carbon usually has 4 valence electrons. In this structure, it has 2 non-bonding electrons (from its lone pair) and half of the 6 bonding electrons (from the triple bond), which is 3. So, 4 - (2 + 3) = -1. Carbon has a formal charge of -1.
Formal Charge on Nitrogen: Nitrogen usually has 5 valence electrons. In this structure, it has 2 non-bonding electrons (from its lone pair) and half of the 6 bonding electrons (from the triple bond), which is 3. So, 5 - (2 + 3) = 0. Nitrogen has a formal charge of 0.
The sum of the formal charges (-1 + 0 = -1) matches the overall charge of the ion, which is -1. This confirms our structure is likely correct. The negative charge on carbon is typical for cyanide, as carbon is slightly less electronegative than nitrogen, so it's more likely to hold onto that extra electron, relatively speaking.
Drawing the CN+ (Cyanonium Ion) Lewis Structure
The cyanonium ion, CN+, is less common than CN-, but it's still a good exercise to understand how losing an electron changes the structure. My text mentions "the lewis structure for cn a + cn a + (cyanonium ion)," so it's definitely part of the discussion.
Valence Electrons for CN+
For CN+, we start with Carbon (4) + Nitrogen (5) and then subtract 1 electron because of the positive charge. So, 4 + 5 - 1 = 8 valence electrons. This is an even number, which again suggests we can likely achieve octets for both atoms, which is good.
Step-by-Step for CN+
Count Total Valence Electrons: We have 8 valence electrons.
Place Atoms and Draw a Single Bond: Connect C and N with a single bond (C-N). This uses 2 electrons, leaving 6 electrons to place.
Distribute Remaining Electrons as Lone Pairs: Give lone pairs to nitrogen first. Add 6 electrons as 3 lone pairs around nitrogen. Nitrogen now has 2 (from bond) + 6 (lone pair) = 8 electrons, completing its octet. We have used all 8 electrons (2 for bond + 6 for lone pairs). Carbon, at this point, only has 2 electrons from the single bond. It needs more, clearly.
Form Multiple Bonds: To give carbon an octet, we need to move lone pairs from nitrogen to form multiple bonds. If we move one lone pair from nitrogen to form a double bond, nitrogen would have 4 electrons in bonds and 4 in lone pairs (2 lone pairs). Carbon would have 4 electrons in bonds. It still needs more. If we move *another* lone pair from nitrogen, forming a triple bond, nitrogen will have 6 electrons in bonds and 2 in a lone pair (1 lone pair). Carbon will have 6 electrons in bonds. Carbon still needs 2 more electrons for an octet. This is where it gets interesting, as a matter of fact.
Checking Octets and Formal Charges for CN+
With only 8 valence electrons, achieving octets for both carbon and nitrogen simultaneously while having a positive charge is a bit of a balancing act. One common and relatively stable structure for CN+ involves a triple bond between C and N, with a lone pair on nitrogen. This would give nitrogen 8 electrons (6 from bond + 2 from lone pair). Carbon would have 6 electrons from the triple bond, but no lone pairs. This leaves carbon with only 6 electrons, making it electron-deficient, which is common for positive ions. This structure is often drawn as C triple bonded to N, with a lone pair on N. The positive charge will reside on the carbon atom, which is pretty common for these sorts of structures.
Formal Charge on Carbon: Carbon usually has 4 valence electrons. In this structure (C≡N with lone pair on N), carbon has 0 non-bonding electrons and half of the 6 bonding electrons, which is 3. So, 4 - (0 + 3) = +1. Carbon has a formal charge of +1.
Formal Charge on Nitrogen: Nitrogen usually has 5 valence electrons. In this structure, it has 2 non-bonding electrons (from its lone pair) and half of the 6 bonding electrons, which is 3. So, 5 - (2 + 3) = 0. Nitrogen has a formal charge of 0.
The sum of the formal charges (+1 + 0 = +1) matches the overall charge of the ion. This structure, where carbon has a positive charge and is electron-deficient, is a plausible representation for the cyanonium ion, CN+, and it's quite different from the cyanide ion, isn't it?
Why These Structures Are So Important
Understanding how to draw Lewis structures for things like the cn lewis structure isn't just a classroom exercise; it's a fundamental skill in chemistry. These simple diagrams tell us so much about a molecule. They show us the bonding patterns, whether atoms are connected by single, double, or triple bonds, and where the lone pairs of electrons are located. This information, in turn, helps us predict a molecule's shape, its polarity, and how it might react with other molecules. It's really the starting point for so much more advanced chemistry, and it's a very practical tool for anyone studying the subject.
My text rightly says that Lewis gave an electron dot representation of a molecule which is helpful in better understanding a molecule and knowing about all the bond pairs and lone pairs present with each. It's true; once you see the electrons, the molecule starts to make a lot more sense. For example, knowing that cyanide (CN-) has a triple bond and a lone pair on carbon helps explain why it's such a potent ligand in coordination chemistry, and it's pretty fascinating how that works, honestly.
Frequently Asked Questions About CN Lewis Structures
How many valence electrons does the cyanide ion (CN-) have?
The cyanide ion, CN-, has a total of 10 valence electrons. This comes from 4 from carbon, 5 from nitrogen, and an additional 1 electron because of its -1 charge. This count is really the key to drawing its Lewis structure correctly, you know.
What is the bond order in CN?
For the neutral CN molecule, because it has an odd number of valence electrons (9 total), it's a bit unique. While not a perfect whole number bond, it's often considered to have a bond order close to 2.5 or 3, depending on the specific model. It's not a simple single, double, or triple bond in the way we usually think of it, which is pretty interesting.
Why is the carbon atom often negative in CN-?
In the cyanide ion (CN-), the negative formal charge typically resides on the carbon atom. This happens even though nitrogen is more electronegative. The reason is that when you draw the most stable Lewis structure with a triple bond and lone pairs on both atoms, placing the lone pair on carbon results in a formal charge of -1 for carbon and 0 for nitrogen. This distribution is more stable because it allows both atoms to achieve an octet, and that's generally a very strong driving force in molecule formation, as a matter of fact. For more general chemistry concepts, you could check out a reputable chemistry resource like the IUPAC website.
- Calia Workout Wear
- Thats Now How It Works Sabrina Chara
- Spongebob Batman
- Steve Haley
- Short Cornrow Braids